Lutetium
| General properties | |
|---|---|
| Name, symbol, number | lutetium, Lu, 71 |
| Element category | lanthanide |
| Group, period, block | n/a, 6, f |
| Standard atomic weight | 174.9668(4) |
| Electron configuration | [Xe] 6s2 4f14 5d1
2, 8, 18, 32, 9, 2 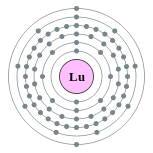 |
| Discovery | Georges Urbain and Carl Auer von Welsbach (1906) |
| First isolation | Carl Auer von Welsbach (1906) |
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal which resists corrosion in dry, but not moist, air. It is the last element in the lanthanide series, and traditionally counted among the rare earths.
Lutetium was independently discovered in 1907 by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James. All of these men found lutetium as an impurity in the mineral ytterbia, which was previously thought to consist entirely of ytterbium. The dispute on the priority of the discovery occurred shortly after, with Urbain and von Welsbach accusing each other of publishing results influenced by the published research of the other; the naming honor went to Urbain as he published his results earlier. He chose the name lutecium for the new element but in 1949 the spelling of element 71 was changed to lutetium. In 1909, the priority was finally granted to Urbain and his names were adopted as official ones; however, the name cassiopeium (or later cassiopium) for element 71 proposed by von Welsbach was used by many German scientists until the 1950s.
Lutetium is not a particularly abundant element, though significantly more common than silver in the earth's crust; it has few specific uses. Lutetium-176 is a relatively abundant (2.5%) radioactive isotope with a half-life of about 38 billion years, and so used to determine the age of meteorites. Lutetium usually occurs in association with the element yttrium and is sometimes used in metal alloys and as a catalyst in various chemical reactions. 177Lu-DOTA-TATE is used for radionuclide therapy (see Nuclear medicine) on neuroendocrine tumours.
Return to Periodic Table
No comments:
Post a Comment