Gallium
| General properties | |
|---|---|
| Name, symbol, number | gallium, Ga, 31 |
| Element category | post-transition metal |
| Group, period, block | 13, 4, p |
| Standard atomic weight | 69.723(1) |
| Electron configuration | [Ar] 4s2 3d10 4p1
2, 8, 18, 3 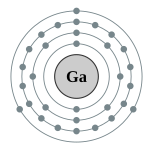 |
| Prediction | Dmitri Mendeleev (1871) | Discovery | Lecoq de Boisbaudran (1875) |
| First isolation | Lecoq de Boisbaudran (1875) |
Gallium is a chemical element with symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium (III) compounds in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. Held long enough, gallium will melt in the hand as it liquefies at temperature of 29.77 °C (85.59 °F) (slightly above room temperature). Its melting point is used as a temperature reference point. The alloy Galinstan (68.5% Ga, 21.5% In, 10% Sn) has an even lower melting point of −19 °C(−2 °F). From its discovery in 1875 until the semiconductor era, gallium was used primarily as an agent to make low-melting alloys.
Today, almost all gallium is used for microelectronics. Gallium arsenide, the primary use of gallium, is used in microwave circuitry and infrared applications. Gallium nitride and indium gallium nitride, minority semiconductor uses, produce blue and violet light-emitting diodes (LEDs) and diode lasers.
Gallium has no known role in biology. Because gallium (III) and ferric salts behave similarly in biological systems, gallium ions often mimic iron ions in medical applications. Gallium-containing pharmaceuticals and radiopharmaceuticals have been developed.
Return to Periodic Table
No comments:
Post a Comment