Mercury
| General properties | |
|---|---|
| Name, symbol, number | mercury, Hg, 80 |
| Element category | transition metal |
| Group, period, block | 12, 6, d |
| Standard atomic weight | 200.59(2) |
| Electron configuration | [Xe] 4f14 5d10 6s2
2, 8, 18, 32, 18, 2 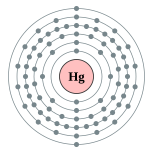 |
| Discovery | Ancient Chinese and Indians (before 2000 BC) |
Mercury is a chemical element with the symbol Hg and atomic number 80. It is commonly known as quicksilver and its scientific name is hydrargyrum (< Greek "hydr-" water and "argyros" silver). A heavy, silvery d-block element, mercury is the only metal that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. With a freezing point of −38.83 °C and boiling point of 356.73 °C, mercury has one of the narrowest ranges of its liquid state of any metal.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is mostly obtained by reduction from cinnabar. Cinnabar is highly toxic by ingestion or inhalation of the dust. Mercury poisoning can also result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury), inhalation of mercury vapor, or eating seafood contaminated with mercury.
Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, and other devices though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely phased out in clinical environments in favor of alcohol-filled, galinstan-filled, digital, or thermistor-based instruments. It remains in use in scientific research applications and in amalgam material for dental restoration. It is used in lighting: electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light which then causes the phosphor in the tube to fluoresce, making visible light.
Return to Periodic Table
No comments:
Post a Comment