Scandium
| General properties | |
|---|---|
| Name, symbol, number | scandium, Sc, 21 |
| Element category | transition metal |
| Group, period, block | 3, 4, d |
| Standard atomic weight | 44.955912(6) |
| Electron configuration | [Ar] 3d1 4s2
2, 8, 9, 2 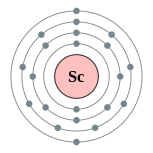 |
| Prediction | Dmitri Mendeleev (1871) |
| Discovery | Lars Fredrik Nilson (1879) |
| First isolation | Lars Fredrik Nilson (1879) |
Scandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic transition metal, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia.
Scandium is present in most of the deposits of rare earth and uranium compounds, but it is extracted from these ores in only a few mines worldwide. Because of the low availability and the difficulties in the preparation of metallic scandium, which was first done in 1937, it took until the 1970s before applications for scandium were developed. The positive effects of scandium on aluminum alloys were discovered in the 1970s, and its use in such alloys remains its only major application. The global trade of the pure metal is around a hundred pounds a year on average.
The properties of scandium compounds are intermediate between those of aluminum and yttrium. A diagonal relationship exists between the behavior of magnesium and scandium, just as there is between beryllium and aluminum. In the chemical compounds of the elements shown as group 3, above, the predominant oxidation state is +3.
Return to Periodic Table
No comments:
Post a Comment