Cadmium
| General properties | |
|---|---|
| Name, symbol, number | cadmium, Cd, 48 |
| Element category | transition metal |
| Group, period, block | 12, 5, d |
| Standard atomic weight | 112.411 |
| Electron configuration | [Kr] 5s2 4d10
2, 8, 18, 18, 2 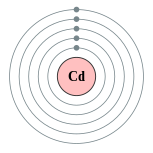 |
| Discovery | Karl Samuel Leberecht Hermann and Friedrich Stromeyer(1817) |
| First isolation | Karl Samuel Leberecht Hermann and Friedrich Stromeyer(1817) |
| Discovery | Friedrich Stromeyer (1817) |
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it prefers oxidation state +2 in most of its compounds and like mercury it shows a low melting point compared to transition metals. Cadmium and its congeners are not always considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states. The average concentration of cadmium in the Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate.
Cadmium occurs as a minor component in most zinc ores and therefore is a byproduct of zinc production. It was used for a long time as a pigment and for corrosion resistant plating on steelwhile cadmium compounds were used to stabilize plastic. With the exception of its use in nickel–cadmium batteries and cadmium telluride solar panels, the use of cadmium is generally decreasing. These declines have been due to competing technologies, cadmium’s toxicity in certain forms and concentration and resulting regulations. Although cadmium has no known biological function in higher organisms, a cadmium-dependent carbonic anhydrase has been found in marine diatoms.
Return to Periodic Table
No comments:
Post a Comment