Darmstadtium
| General properties | |
|---|---|
| Name, symbol, number | darmstadtium, Ds, 110 |
| Element category | unknown but probably a transition metal |
| Group, period, block | 10, 7, d |
| Standard atomic weight | (281) |
| Electron configuration | [Rn] 5f14 6d8 7s2
(predicted) 2, 8, 18, 32, 32, 16, 2 (predicted) 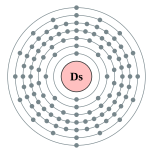 |
| Discovery | Gesellschaft für Schwerionenforschung (1994) |
Darmstadtium is a chemical element with the symbol Ds and atomic number 110. It is an extremely radioactive synthetic element. The most stable known isotope, darmstadtium-281, has a half-life of approximately 11 seconds, but it is possible that this darmstadtium isotope may have an isomer with a longer half-life, 3.7 minutes. Darmstadtium was first created in 1994 by the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt-Arheilgen near Darmstadt, Germany. It was named after the city of Darmstadt, where it was discovered.
In the periodic table, it is a d-block transactinide element. It is a member of the 7th period and is placed in the group 10 elements, although no chemical experiments have yet been carried out to confirm that it behaves as the heavier homologue to platinum in group 10. Darmstadtium is calculated to have similar properties to its lighter homologues, nickel, palladium, and platinum.
Return to Periodic Table
No comments:
Post a Comment