Bismuth
| General properties | |
|---|---|
| Name, symbol, number | lbismuth, Bi, 83 |
| Element category | post-transition metal |
| Group, period, block | 15, 6, p |
| Standard atomic weight | 208.98040(1) |
| Electron configuration | [Xe] 4f14 5d10 6s2 6p3
2, 8, 18, 32, 18, 5 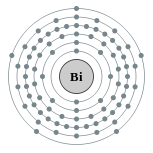 |
| Discovery | Claude François Geoffroy (1753) |
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a pentavalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery white color when freshly produced, but is often seen in air with a pink tinge owing to surface oxidation. Bismuth is the most naturally diamagnetic and has one of the lowest values of thermal conductivity among metals.
Bismuth metal has been known from ancient times, although until the 18th century it was often confused with lead and tin, which share some physical properties. The etymology is uncertain, but possibly comes from Arabic bi ismid, meaning having the properties of antimony or German words weisse masse or wismuth ("white mass"), translated in the mid sixteenth century to New Latin bisemutum.
Bismuth has long been considered as the highest-atomic-mass element that is stable. However, it was recently discovered to be slightly radioactive: its only primordial isotopebismuth-209 alpha decays with a half life more than a billion times the estimated age of the universe.
Bismuth compounds account for about half the production of bismuth. They are used in cosmetics, pigments, and a few pharmaceuticals, notably Pepto-Bismol. Bismuth has unusually low toxicity for a heavy metal. As the toxicity of lead has become more apparent in recent years, there is an increasing use of bismuth alloys (presently about a third of bismuth production) as a replacement for lead.
Return to Periodic Table
No comments:
Post a Comment